Optomed has received a regulatory approval for Optomed Aurora in China
Optomed Plc, Inside Information, 11 November 2020 at 9.30, Helsinki
Optomed has received a regulatory approval for Optomed Aurora in China
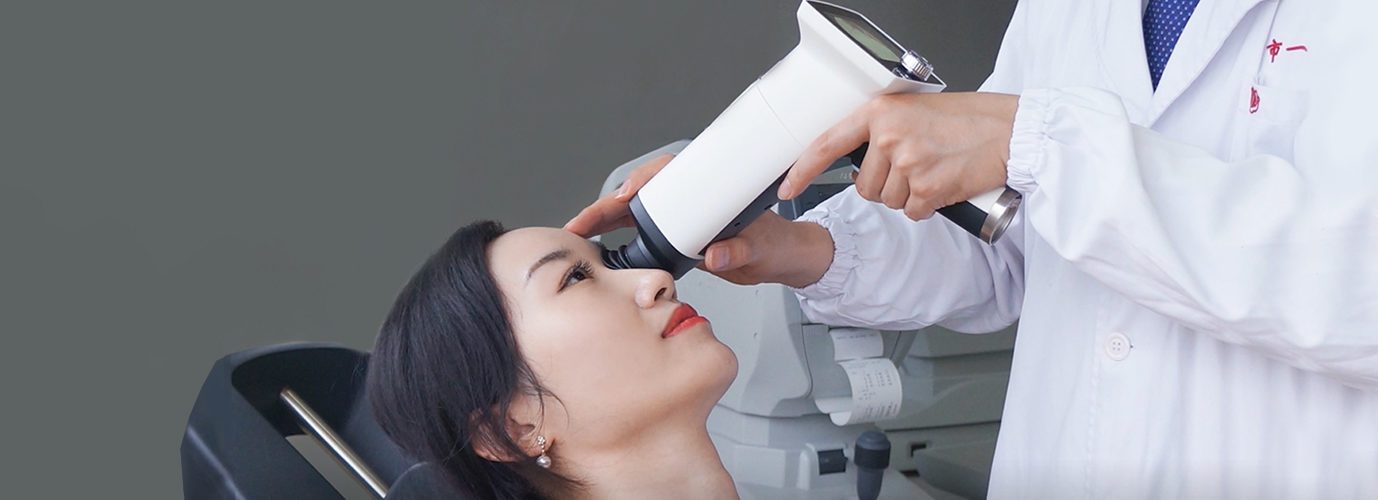
Optomed has received a medical devices regulatory approval in the People’s Republic of China for Optomed’s latest generation handheld camera, Optomed Aurora.
The Chinese Food and Drug Administration (CFDA) approval allows Optomed to start commercial operations of Optomed Aurora in the People’s Republic of China. Prior to this approval, Optomed has only been able to sell its previous generation camera, Smartscope Pro, in China.
Optomed Aurora is made for examination and documentation of the retina for various eye diseases and neurological disorders. The Aurora camera is non-mydriatic, which means there is no need for pupil dilatation when imaging the fundus. It has a 50-degree field-of-view which is useful especially in diabetic retinopathy screening. The Aurora camera can be easily integrated into different hospital systems.
Laura Piila, Optomed’s Vice President Devices comments: “First, I would like to thank everybody involved with the approval process. The approval enables us to provide a camera that represents our latest technology to our Chinese customers which again helps to prevent avoidable blindness and reduce overall healthcare costs in China. All internal launch preparations are completed, so sales activities can start immediately”.
Optomed Plc
Further enquiries
Seppo Kopsala,
CEO, Optomed Plc, seppo.kopsala@optomed.com
Laura Piila,
Vice President Devices, Optomed Plc, +358 40 588 1187, laura.piila@optomed.com
Optomed in Brief
Optomed is a Finnish medical technology company and one of the leading providers of handheld fundus cameras. Optomed combines handheld cameras with software and artificial intelligence with the aim to transform the diagnostic process of blinding eye-diseases such as rapidly increasing diabetic retinopathy. In its business Optomed focuses on eye-screening devices and software solutions related R&D in Finland and sales through different channels in over 60 countries. The company has an extensive portfolio of 55 international patents protecting the technology. In 2019, Optomed’s revenue reached EUR 15 million and at the end of 2019 Optomed employed 108 professionals.